Below are the main questions in organismal growth biology that the Biga Lab works on – starting with currently active projects.
Are maternal dietary effects on offspring growth due to epigenetic mechanisms? USDA-Funded AFRI Project
Based on our work with MR, HM, and growth regulation, we considered an alternative hypothesis that methionine supplementation would improve trout growth through similar pathways (GH/IGF, mTOR, etc.). Since evidence shows that supplementing salmonid diets with methionine (or other methyl-donating amino acids) has no effect on muscle growth, we hypothesized that supplementing broodstock with methyl-donating amino acids could alter offspring growth via epigenetic profiles. In collaboration with Dr. Beth Cleveland (USDA, ARS, Leetown, WV USA) we demonstrated that supplementing maternal broodstock diets with choline results in enhanced offspring growth performance (Fig. 1)1.

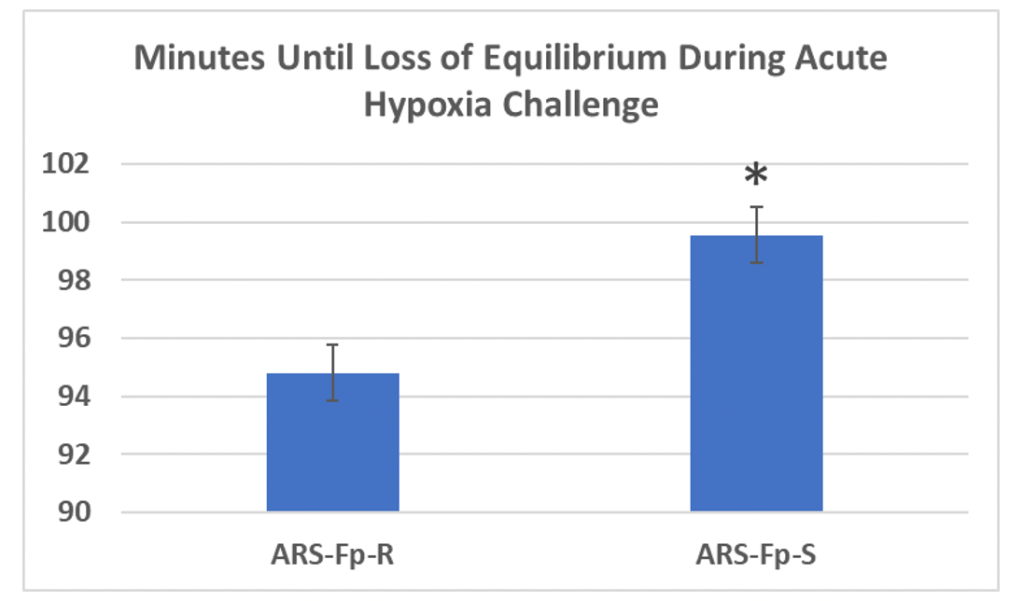
This work continued with support from USDA NIFA AFRI (#2018-67015-27478) where we demonstrated a strong genetic effect of response to maternal diet, and identified differentially regulated genes (116 DEGs) in 0 and 14-day post hatch offspring from choline supplemented dams2. Additionally, we identified 79 hypermethylated and 146 hypomethylated gene regions in 0-day post-hatch offspring from choline-supplemented dams (manuscript in prep). Upon integration of the RNAseq and RRBS datasets (comparing DEGs and differentially methylated DNA sites), we identified common gene regions from choline-supplemented and non-choline-supplemented groups. For brevity, Hsp90 and Hif-1alpha were identified as hypomethylated gene regions and as upregulated genes in offspring from dams not supplemented with choline. This data led to our current USDA NIFA AFRI funding (#2023-67016-39339) focused on understanding 1) how genetic selection for particulate performance traits (fillet yield or disease resistance) affects other important phenotypes like hypoxia tolerance, and 2) how maternal dietary choline intake and hypoxia exposure affect offspring growth and hypoxia tolerance. In a preliminary study, we showed that the disease-resistant selected families lose equilibrium faster than their disease susceptible counterparts (Fig. 2), suggesting a negative effect from single trait selection. Our current grant is evaluating this effect across more families and the fillet yield selected line to investigate whether maternal diet and/or acute hypoxia exposure during oogenesis programs hypoxia tolerance through epigenetic marks.
- Cleveland, B. M. et al. Supplementing rainbow trout ( Oncorhynchus mykiss ) broodstock diets with choline and methionine improves growth in offspring. J World Aquacult Soc 51, 266–281 (2020).
- Freij, K., Cleveland, B. & Biga, P. Maternal dietary choline levels cause transcriptome shift due to genotype-by-diet interactions in rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 49, 101193 (2024).
What mechanisms regulate sex-specific aging phenotypes? NSF-Funded Biology Integration Institute: IISAGE

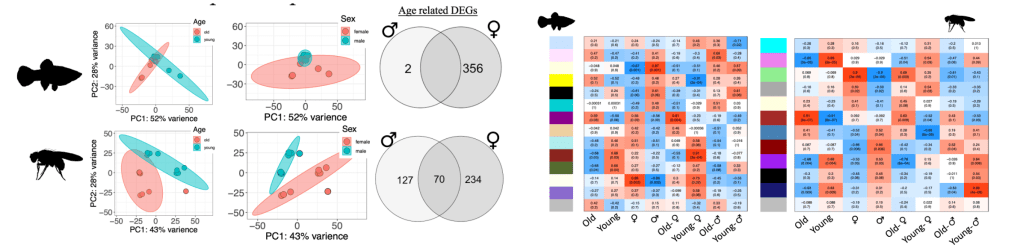
More recently, we began to focus on Poecilid fishes, as several species exhibit sexually dimorphic growth patterns, where one sex grows larger and faster than the other and this growth resembles indeterminate growth. The opposite sex appears to exhibit determinate muscle growth, thus providing a valuable comparative model system within individual species. Poecilid fishes are diverse in their phenotypes, where in some species the females grow larger and faster than the males, while in other species the male grows larger and faster than the females. Also, some species do not exhibit sexually dimorphic growth at all. Specifically, Xiphophorus species provide us with a unique opportunity to evaluate highly related growth-regulating pathways in divergent phenotypes. In addition, these growth characteristics are also highly correlated to aging phenotypes, where faster-growing individuals tend to live shorter lives compared to slower-growing counterparts. Our work with Poecilid fishes is part of a large multi-institution Biology Integration Institute project funded through an NSF cooperative agreement (DBI-2213824, Co-PI) – Integration Institute: Sex, Aging, Genomics, and Evolution (IISAGE – https://www.iisage.org/). We are testing mechanistic hypotheses for variation in sex-specific aging that derive from genetics (e.g., sex-specific genomics, epigenomes), organismal biology (e.g., specific cellular stress responses, body size), quantitative genetics (e.g., sex-specific phenotypic plasticity), and evolutionary biology (e.g., macro-evolutionary patterns and processes) across 64 species by collecting matched samples (male, female, old, young) for comparative genomic and epigenomic analyses (RNAseq, ATACseq, Cut&Run or RRBS/WGBS) and cellular aging phenotype measures (DNA repair efficiency, mitochondrial function). The Biga Lab’s role in IISAGE related to research includes all things fish, where we will compare multiple Xiphophorus and Heterandria species (male, female, old, young) aging phenotypes in relation to numerous sexually dimorphic phenotypes (like growth) and will test any mechanisms identified in medaka, as Xiphophorus and Heterandria are live-bearing fish while medaka are closely related but more amenable to genetic manipulations. We have currently sampled 3 fish species for RNAseq, ATACseq, and RRBS analyses, with data in various stages of processing. Figure 3 outlines comparative genomic analyses we are working on from Xiphophorus maculatus and Drosophila melanogaster RNAseq data comparing old, young, male, female animals. We are also validating the zebrafish DNA methylation aging clock for use with Xiphophorus and Heterandria for accurate aging from lab colonies and wild-caught populations.
The IISAGE work related to fish species is conducted by the Biga Laboratory in collaboration with several labs across the world: Dr. Molly Morris (Ohio University), Dr. Frauke Seeman (Texas A&M Corpus Christie), Dr. Oscar Rios-Cardenas (Instituto de Ecologia, Veracruz, MX), and Dr. Ken Cain (NOAA, Manchester, WA). The lab is also collaborating with Dr. Mike Sandel (Mississippi State University) and Dr. Zach Culumber (University of Alabama in Huntsville) to understand the contribution of sex-linked genes to variation in growth and aging in Xiphophorus sp. with particular interest in the Mc4r gene that is related to age at maturity and body size.
Review Articles from IISAGE
- Riddle, N.C., P.R. Biga, A.M. Bronikowski, J.R. Walters, G.S. Wilkinson, and IISAGE Consortium. 2023. Comparative analysis of animal lifespan. GeroScience. https://doi.org/10.1007/s11357-023-00984-2.
- Bronikowski, A. M., Meisel, R. P., Biga, P. R., Walters, J. R., Mank, J. E., Larschan, E., Wilkinson, G. S., Valenzuela, N., Conard, A. M., de Magalhães, J. P., Duan, J., Elias, A. E., Gamble, T., Graze, R. M., Gribble, K. E., Kreiling, J. A., and Riddle, N. C. (2021). Sex-specific aging in animals: Perspective and future directions. Aging Cell, 00, e13542. https://doi.org/10.1111/acel.13542
Does Growth Hormone have Direct Action in Peripheral Tissues.
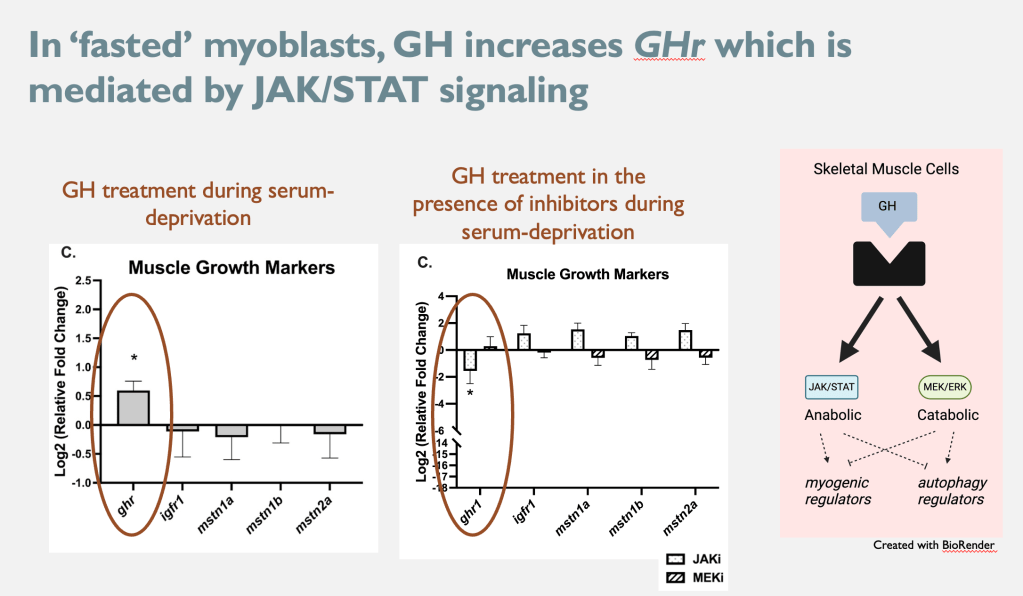
Muscle-specific growth in fish has been the focus of many aquaculture studies, as fish skeletal muscle growth has significant economic implications on production efficiency and product yield. Decades of growth-focused research has improved our understanding of how nutrition, the environment, and the endocrine system affect molecular mechanisms regulating skeletal muscle growth in fishes. The endocrine system plays a key role in regulating muscle growth via the actions of the growth hormone (GH)-insulin-like growth factor-1 (IGF-1) system. The general consensus of the GH/IGF-I system, is that GH turns IGF-I production on (mainly in the liver), and IGF-I then mediates the growth-regulating functions of GH. These actions of GH are elicited through its receptor (GHr), which can activate anabolic (JAK/STAT) or catabolic (MEK/ERK) signaling pathways. Skeletal muscle tissue exhibits dynamic GHr expression that is sensitive to physiological conditions. However, there is a limited understanding of what actions GH might elicit on muscle tissue that are not mediated by IGF-I. We recently tested GH action in an in vitro model where IGF-I signaling is limited. Figure 4 shows that during a ‘fasted’ condition where IGF-I is limited, GH induces the expression of GHr (its own receptor) via the JAK/STAT signaling pathway1.
This work is ongoing, in collaboration with Dr. Beth Cleveland (USDA, ARS, NCCCWA) and Dr. Munetaka Shimizu (Hokkaido University, Sapporo, Japan). We plan to optimize the in vitro system to better mimic in vivo fasting conditions to have a tool to analyze the role of GH signaling at the level of muscle tissue throughout myogenic ontology. Additionally, we will analyze the role of IGF-I, IGF-II, and the multiple salmonid IGF binding proteins.
- Reid, R.M., S. Turkmen, B.M. Cleveland, and P.R. Biga. 2024. Direct actions of growth hormone in rainbow trout, Oncorhynchus mykiss, skeletal muscle cells in vitro. Comp. Biochem. and Phys., Part A. Mol. and Int. Phys. 297:111725. https://doi.org/10.1016/j.cbpa.2024.111725
What role does myostatin play in metabolic regulation in fish species?
Much of my early work focused on the growth hormone/insulin-like growth factor (GH/IGF) pathway and the peptide myostatin (MSTN) where we established unique functions and regulation of these factors in indeterminate growing teleost species1–5. MSTN has been shown to negatively regulate cell proliferation and differentiation and is mostly muscle-specific in mammals. Teleost fish express multiple copies of MSTN due to whole genome duplication events – rainbow trout express MSTN1a, MSTN1b, MSTN2a, and MSTN2b. These trout isoforms are differentially expressed in many trout tissues, even MSTN2b which contains an early stop codon. Our work has shown various unique aspects of MSTN regulation, including in response to nutrient availability and stress6-9. We also showed that in mice, MSTN is expressed in spleen tissue and is upregulated via fasting or stress10, suggesting MSTN functions outside muscle cell regulation and serves as a regulator of metabolic pathways11. Additionally, we hypothesize that MSTN2b in trout has likely evolved a novel function. We continue to investigate the role of MSTN in metabolic regulation and identifying the novel function of MSTN2b in trout and I am currently building a collaboration with Dr. Micheal Phelps (Washington State University).
- Biga, P. R. & Goetz, F. W. Zebrafish and giant danio as models for muscle growth: determinate vs. indeterminate growth as determined by morphometric analysis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 291, R1327–R1337 (2006).
- Biga, P. R. & Meyer, J. Growth hormone differentially regulates growth and growth-related gene expression in closely related fish species. Comp Biochem Physiol A Mol Integr Physiol 154, 465–473 (2009).
- Biga, P. R. et al. The isolation, characterization, and expression of a novel GDF11 gene and a second myostatin form in zebrafish, Danio rerio. Comp Biochem Physiol B Biochem Mol Biol 141, 218–230 (2005).
- Biga, P. R. et al. Growth hormone differentially regulates muscle myostatin1 and -2 and increases circulating cortisol in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 138, 32–41 (2004).
- Biga, P. R. et al. The effects of recombinant bovine somatotropin (rbST) on tissue IGF-I, IGF-I receptor, and GH mRNA levels in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 135, 324–333 (2004).
- Galt, N. J., McCormick, S. D., Froehlich, J. M. & Biga, P. R. A comparative examination of cortisol effects on muscle myostatin and HSP90 gene expression in salmonids. Gen Comp Endocrinol 237, 19–26 (2016).
- Galt, N. J., Froehlich, J. M., Remily, E. A., Romero, S. R. & Biga, P. R. The effects of exogenous cortisol on myostatin transcription in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol A Mol Integr Physiol 175, 57–63 (2014).
- Galt, N. J., Froehlich, J. M., Meyer, B. M., Barrows, F. T. & Biga, P. R. High-fat diet reduces local myostatin-1 paralog expression and alters skeletal muscle lipid content in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 40, 875–886 (2014).
- Meyer, B. M., Froehlich, J. M., Galt, N. J. & Biga, P. R. Inbred strains of zebrafish exhibit variation in growth performance and myostatin expression following fasting. Comp Biochem Physiol A Mol Integr Physiol 164, 1–9 (2013).
- Lyons, J.-A., Haring, J. S. & Biga, P. R. Myostatin expression, lymphocyte population, and potential cytokine production correlate with predisposition to high-fat diet induced obesity in mice. PLoS One 5, e12928 (2010).
- Gabillard, J.-C., Biga, P. R., Rescan, P.-Y. & Seiliez, I. Revisiting the paradigm of myostatin in vertebrates: insights from fishes. Gen Comp Endocrinol194, 45–54 (2013).
Is muscle growth regulation due to epigenetic mechanisms?
To understand the molecular regulation of growth, we characterized epigenetic mechanisms regulating muscle differentiation in rainbow trout. Working closely with Dr. Jean-Charles Gabillard (INRA, Rennes, France) and Dr. Iban Seiliez (INRA, St. Pee, France) we characterized the histone methylation profile related to pax7 and myogenin expression during in vitro myogenesis in rainbow trout, an indeterminate growing fish1. Additionally, we demonstrated that methionine depletion specifically alters this epigenetic profile, as well as reverts myoblasts to the quiescent state, suggesting a role of histone methylation (HM) in the regulation of myogenic progression. This quiescence appears to be reversible with the addition of methionine, and the methionine-induced myogenic differentiation is associated with microRNA-2102. We also demonstrated, in vivo, that methionine restriction (MR) affects glucose tolerance, lipid deposition, and microRNA expression in skeletal muscle3 and glucose availability regulates protein turnover4. To further analyze muscle cell regulation, we characterized a novel zebrafish in vitro model of autophagy that utilizes amino acid-depleted media to induce autophagy without apoptosis during myogenesis5. We characterized the HM profiles affected by this cell phenotype switch and identified Atg4b, p62/sqstrm1, and lc3b as tightly regulated by starvation during the onset of autophagy. Currently, this work is being continued by a current PhD student (Michael Addo) who has shown that MR is likely regulating myogenic progression through circadian clock genes via DNA methylation and miRNA regulation.
- Seiliez, I., Froehlich, J. M., Marandel, L., Gabillard, J.-C. & Biga, P. R. Evolutionary history and epigenetic regulation of the three paralogous pax7 genes in rainbow trout. Cell Tissue Res 359, 715–727 (2015).
- Latimer, M. et al. miR-210 expression is associated with methionine-induced differentiation of trout satellite cells. Journal of Experimental Biology jeb.154484 (2017) doi:10.1242/jeb.154484.
- Latimer, M. N., Cleveland, B. M. & Biga, P. R. Dietary methionine restriction: Effects on glucose tolerance, lipid content and micro-RNA composition in the muscle of rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 208, 47–52 (2018).
- Latimer, M. N., Reid, R. M., Biga, P. R. & Cleveland, B. M. Glucose regulates protein turnover and growth-related mechanisms in rainbow trout myogenic precursor cells. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 232, 91–97 (2019).
- Biga, P. R., Latimer, M. N., Froehlich, J. M., Gabillard, J.-C. & Seiliez, I. Distribution of H3K27me3, H3K9me3, and H3K4me3 along autophagy-related genes highly expressed in starved zebrafish myotubes. Biol Open 6, 1720–1725 (2017).
Why can most fish species continue to grow throughout their lives?
Unique myogenic precursor cells in indeterminately growing fish muscle – Adult hyperplastic muscle growth, in the absence of trauma or injury, is responsible for continued muscle growth and the ability to reach large adult sizes in indeterminately growing vertebrates. We demonstrated that indeterminate-growing fish exhibit satellite cells that express high levels of Pax3, corresponding to enhanced proliferation capacity compared to muscle satellite cells from determinately growing fish (Fig. 5)1,2. In determinate growth, Pax3 expression is highest during embryonic development with limited to no expression post birth. We hypothesize that muscle satellite cells from indeterminate growing species exhibit a unique embryonic-like phenotype compared to a more committed phenotype observed in determinately growing vertebrates, like mammals. We are now investigating whether changing Pax3 expression can alter this cellular phenotype and whether this expression is related to age-related muscle wasting resistance often observed in indeterminately growing fish species. Additionally, we are investigating whether Pax3 expression correlated to sex-specific aging phenotypes in relation to sexually dimorphic growth phenotypes as part of IISAGE.

- Froehlich, J. M., Galt, N. J., Charging, M. J., Meyer, B. M. & Biga, P. R. In vitro indeterminate teleost myogenesis appears to be dependent on Pax3. In Vitro Cell Dev Biol Anim 49, 371–385 (2013).
- Froehlich, J. M., Fowler, Z. G., Galt, N. J., Smith, D. L. & Biga, P. R. Sarcopenia and piscines: the case for indeterminate-growing fish as unique genetic model organisms in aging and longevity research. Front Genet4, 159 (2013).
Can Teneurin C-terminal Associated Peptide (TCAP) improve muscle function and metabolism across animals?
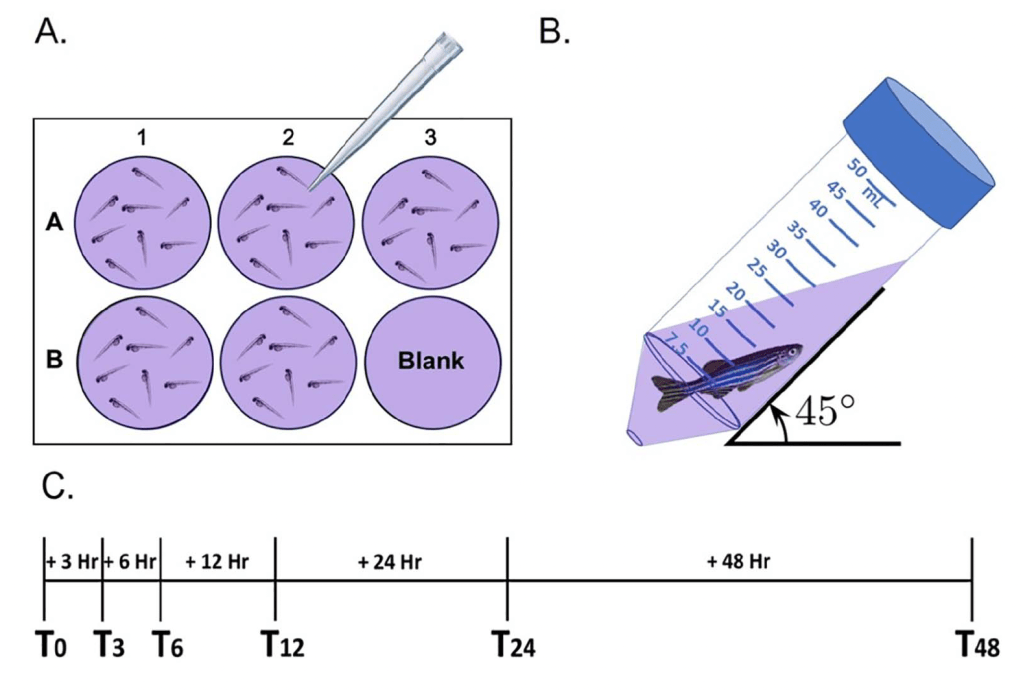
An active peptide cleaved from the c-terminus of teneurin proteins, TCAP, is known to regulate the stress axis, neurogenesis, cytoskeletal arrangement, and calcium flux in rodents. In collaboration with Dr. David Lovejoy (University of Toronto, Canada) we demonstrated the conserved role TCAP-3 plays in metabolic activity in zebrafish1-2 and in muscle activity3. For this work, we also validated a non-invasive and low-input methodology for measuring oxygen consumption over time1,4. Figure 6 depicts the base setup for this method. We hypothesize that TCAP-3 can reduce the onset of age-related muscle pathology, like calcium and ROS buildup, inefficient ATP production, and cytoskeletal breakdown. We continue to evaluate the importance of TCAP-3 in muscle regulation and plan to incorporate this work into our NSF-funded IISAGE work.
- Reid, R. M., Reid, A. L., Lovejoy, D. A. & Biga, P. R. Teneurin C-Terminal Associated Peptide (TCAP)-3 Increases Metabolic Activity in Zebrafish. Front. Mar. Sci. 7, 591160 (2021).
- Reid, R. M., Freij, K. W., Maples, J. C. & Biga, P. R. Teneurins and Teneurin C-Terminal Associated Peptide (TCAP) in Metabolism: What’s Known in Fish? Front Neurosci 13, 177 (2019).
- Hogg, D. W. et al. Skeletal muscle metabolism and contraction performance regulation by teneurin C-terminal-associated peptide-1. Front. Physiol. 13, 1031264 (2022).
- Reid, R. M., D’Aquila, A. L. & Biga, P. R. The validation of a sensitive, non-toxic in vivo metabolic assay applicable across zebrafish life stages. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 208, 29–37 (2018).
